Single molecule force spectroscopy unravels the complex dynamics of HSP70-induced protein conformation
A new way to study protein modifications and the associated chaperones that protect the protein from non-cognate interactions could help us understand what actually drives the modifications. Such an understanding could help explain the progression of diseases such as cancer, Parkinson's and Alzheimer's.
Proteins are involved in almost every process in a cell. This requires them to adopt a precisely defined 3-D structure to carry out their specified function, called 'conformational conformation'. But during this time, a variety of conditions such as chemical, environmental and physical stress can cause protein molecules to fold or unfold incorrectly, which leads to their inability to perform their functions properly. This results in the accumulation of toxins in the cell, leading to diseases. Neuro-degenerative diseases that debilitate the nervous system such as Alzheimer's or Parkinson's have also been linked to the accumulation of toxins in cells.
While many newly modified proteins can fold on their own, many of them need the help of molecular chaperones to regain their native form and avoid non-cognate interactions. The chaperones that protect the molecules are essential for the proper functioning of protein molecules. They not only help them fold, but also correct their unfolding and misfolding.
Given the importance of molecular chaperones in our health, researchers are also studying their structure and function in our cells. Bulk biochemical measurements provide information about protein folding efficiency and protection against aggregation when chaperones are present during protein folding.
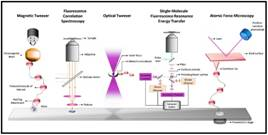
However, conventional single-molecule experiments cannot investigate the diversity of chaperone molecules and how each of these molecules functions in an individual cell. Also, short-term changes in the state of the chaperones are not amenable to such single-molecule experiments. The importance of such temporary states in food digestion processes is poorly understood. In recent years, the development of single-molecule techniques has opened new avenues to explore the fundamental properties of biomolecules involved in a variety of biochemical reactions.
A team from S.N. Bose National Centre for Basic Sciences (an autonomous institute of DST) led by Prof. Shubhashish Haldar is using covalent magnetic tweezers (CMT) designed in their lab to study the physical and chemical properties of protein molecules and the action of chaperones during the folding and functioning of the molecules.
This new approach has provided unexpected insights into the complex process of chaperone-assisted protein folding. The key players in this entire ballet of molecules are the heat shock proteins Hsp70 and Hsp90, two of the most studied molecular chaperones.
Researchers are now beginning to understand how Alzheimer's begins due to brain stiffness. When the physical basis of degenerative diseases such as Alzheimer's and Parkinson's is understood at the molecular level, drugs can be designed to target the mechanistic roles of chaperones. This will make it easier to stop the progression of these diseases.